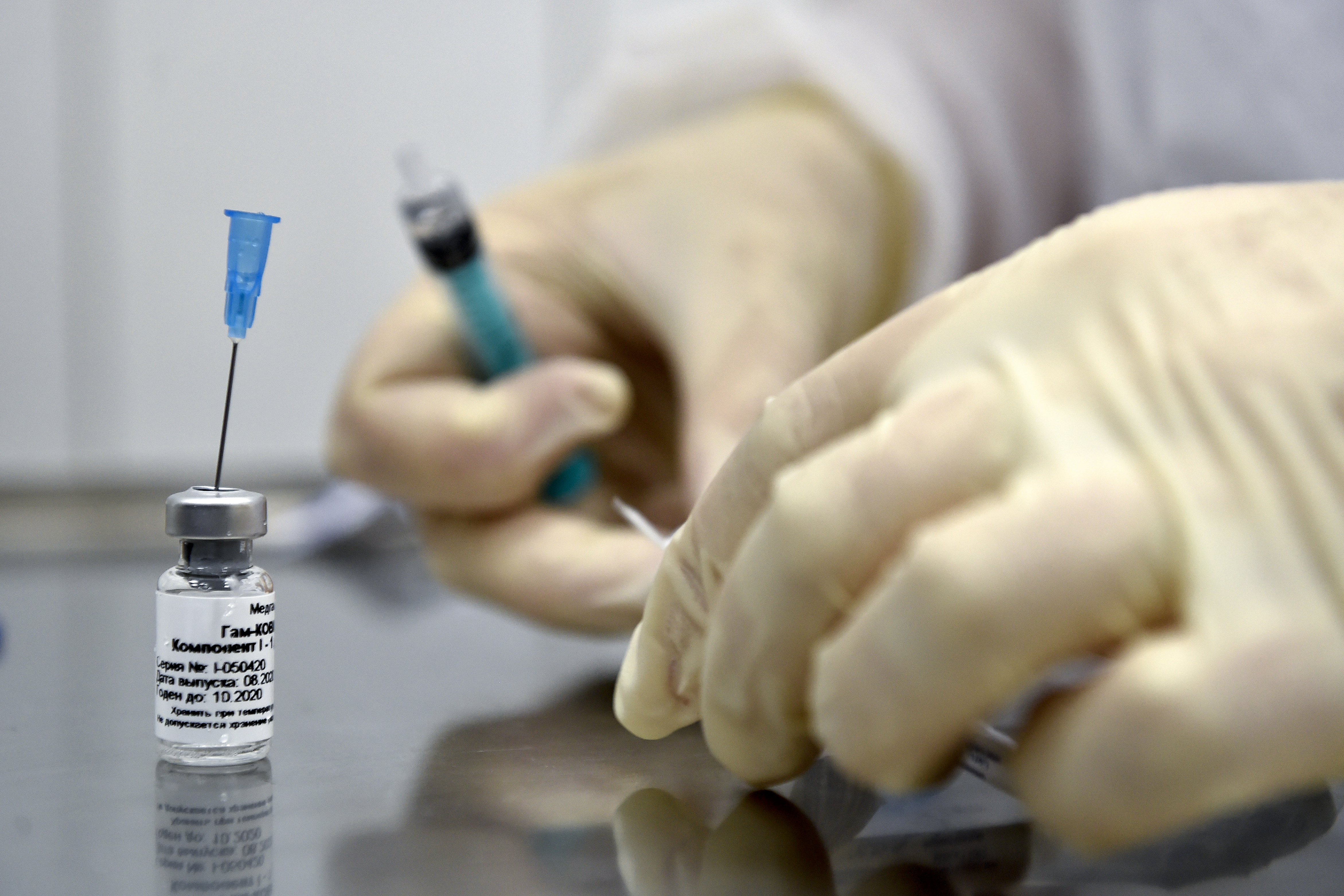
The first batch of the Sputnik V vaccine against the novel coronavirus, developed by the Russian research institution Gamaleya Research Institute of Epidemiology and Microbiology, arrived in Venezuela to start phase three of clinical trials on 2,000 local patients.
While the executive vice president, Delcy Rodríguez, described Venezuela's participation in phase three of the clinical trials as a "historic moment," Western scientists have questioned the Russian government's accelerated authorization process for the vaccine.
Russian president Vladimir Putin approved the Sputnik V vaccine on August 11, 2020, three weeks before the results of phases one and two of the clinical trials were published, and before phase three was started, which is essential in the determination of the security and efficiency of the vaccine. On the date the vaccine was approved, it had only been tested on 76 people.
As reported by Science Magazine, the registration certificate for Sputnik V permits the application of the vaccine on "a small group of citizens from vulnerable groups." The certificate indicates that the vaccine cannot be used until January 2021, which would give it enough time to complete phase three of the clinical trials.
Generally, the authorization of a vaccine requires it to successfully pass phase three of the clinical trials, which includes tens of thousands of participants. Johnson & Johnson, for example, is conducting the larger clinical trials of phase three on 60,000 volunteers in order to get its product authorized.
To continue reading in Spanish, click here.