In January, the director-general of the World Health Organization (WHO), Tedros Adhanom Ghebreyesus, issued a blunt warning. The world was “on the brink of a catastrophic moral failure,” he said. Wealthy countries were buying up available COVID-19 vaccines, leaving tiny amounts for others—a replay of what happened during the 2009 influenza pandemic. “The price of this failure will be paid with lives and livelihoods in the world’s poorest countries,” Tedros said.
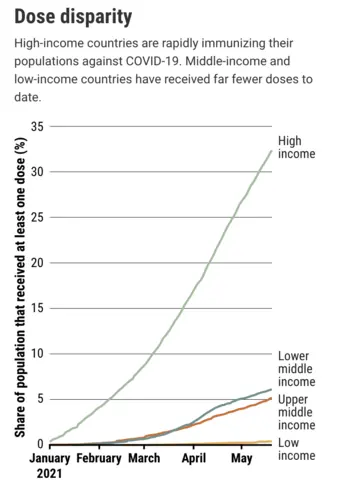
He was right. Today, some rich countries are vaccinating children as young as 12 years old, who are at extremely low risk of developing severe COVID-19, while poorer countries don’t even have enough shots for health care workers. Nearly 85% of the COVID-19 vaccine doses administered to date have gone to people in high-income and upper middle–income countries. The countries with the lowest gross domestic product per capita only have 0.3%.
Tedros lambasted the “scandalous inequity” again in his opening speech at the World Health Assembly on 24 May. By September, at least 10% of the population in every country should be vaccinated, he said.
Disparities in global health are nothing new. Lifesaving therapies such as monoclonal antibodies are unavailable in large parts of the world. Even vaccines and drugs that cost pennies to make don’t reach millions of people who need them. But the COVID-19 crisis has exposed the inequities in a distinct, acute way. As normality is returning to vaccine front-runners such as Israel, the United Kingdom, and the United States, India’s health system is buckling under soaring case numbers—and the world is still recording almost 5 million cases and more than 80,000 deaths every week.
The moral argument aside, there’s a very practical reason to try to distribute vaccines more equitably: No part of the world can feel safe if the pandemic rages on elsewhere, posing the risk of reintroduction and spawning potentially more dangerous viral mutants.
Things might get better. The COVID-19 Vaccines Global Access (COVAX) Facility, a nonprofit, is purchasing shots in bulk at a discount and distributing them to the world’s most resource-strapped countries. Although it’s in major trouble just now, in part because its main supplier, based in India, is reneging on its promises, there is reason to be hopeful: Many rich countries will soon have big stashes of superfluous vaccines—1 billion doses 4 months from now, by one projection—that they could donate to countries in need.
Production capacity is increasing rapidly, too. Some projections suggest enough vaccine could be available this year to give every person in the world at least one dose, although getting them to every country and into every arm would still be a major challenge. For the longer term, plans are now afoot to build vaccine production facilities in more regions of the world to pump up supplies, both for this pandemic and future ones.
Here’s a look at all of these efforts—and when they might offer relief.
WEEKS TO MONTHS
COVAX
COVAX was formed in April 2020 to avoid exactly the scenario playing out now. Jointly run by WHO, the Coalition for Epidemic Preparedness Innovations (CEPI), and Gavi, the Vaccine Alliance, the organization aimed to bring together countries to invest in several vaccine candidates that it would then distribute equitably among participants. High-income countries, companies, and philanthropic organizations would foot the bill for the 92 poorest countries.
At first it appeared to be working: Almost every country in the world has signed up and COVAX began to deliver its first vaccine doses on 24 February, just 2 months after vaccinations started in Europe. But COVAX lacked the money to compete with rich countries that cornered the market early on by striking purchase deals with vaccinemakers. “It was slower than anyone would have wanted in making deals,” says Nicole Lurie, U.S. director of CEPI. “That’s been a real frustration.”
Another major blow came in March, when skyrocketing COVID-19 cases at home led the Serum Institute of India—which COVAX banked on as its main supplier—to halt exports of its vaccine, made in collaboration with AstraZeneca and the University of Oxford. Exports may not resume until the end of the year. “We’re in a bit of a crisis,” says Seth Berkley, who heads Gavi. COVAX will be 190 million doses short by the end of June. “We’re now trying to fill that hole,” he says. As of 23 May, 125 countries had received just 68 million vaccine doses from COVAX.
Serum could resume shipments earlier than December, Berkley says, but if it doesn’t, COVAX could be short 1 billion doses by the end of this year. “What we thought COVAX would prevent is happening,” acknowledges WHO Chief Scientist Soumya Swaminathan.
Vaccine donations from rich countries could help. Because it was unclear in 2020 which vaccines might work, many countries made major deals with multiple companies; the United States and the European Union advance purchased enough to vaccinate their populations three times over, for example. Although not all of those doses have arrived, the United States already has a surplus, and Europe should have enough vaccine for all its residents this summer.
Wealthy countries should donate 1 billion doses to COVAX by 1 September and another billion by mid-2022, contends a report published in early May by the Independent Panel for Pandemic Preparedness and Response (IPPPR). Mark Dybul, former head of the U.S. government’s President’s Emergency Plan for AIDS Relief and a member of IPPPR, says this is urgent. “We need to really press and accelerate and get all the excess surplus out as rapidly as possible,” he says.
Many rich countries, concerned about viral variants and new outbreaks, are hesitant to give up vaccines they might still need, Berkley says: “There’s a lot of unknowns at the moment that make people nervous.” But donations have already begun. Norway and New Zealand have both given the vaccine they were eligible to receive through COVAX back to the facility. Some European countries are ready to donate stocks of AstraZeneca-Oxford shots, whose use there was recently restricted to older age groups because they can cause a rare but serious clotting disorder. The biggest pledge so far comes from the Biden administration, which aims to donate 80 million doses by the end of June, including 60 million doses of the AstraZeneca-Oxford vaccine.
There’s also hope that COVAX may add more vaccines to its arsenal. It is relying heavily on AstraZeneca and its partners because their adenovirus-based vaccine is cheap and, unlike messenger RNA (mRNA) vaccines, can be transported in regular refrigerators. Still, Pfizer and its partner BioNTech in January agreed to sell COVAX up to 40 million doses of its mRNA vaccine this year. On 3 May, Moderna followed with a deal to sell 34 million doses of its mRNA shot this year and 444 million in 2022. Both companies said they will give COVAX a discount, but didn’t reveal their price.
At the World Health Assembly, Tedros made a bold call for all manufacturers to offer any new product to COVAX before putting it on the market, or to commit 50% of their doses to the facility.
MONTHS
Expanding production
To date, all manufacturers combined have distributed fewer than 2 billion doses of COVID-19 vaccines, most of which require two shots. But much more vaccine is on its way. Many of the 14 manufacturers of authorized products continue to build new plants, contract with other manufacturers, and iron out production glitches.
Pfizer and BioNTech hope to produce about 3 billion doses by year’s end, one-third of which they plan to offer to COVAX or directly to low- and middle-income countries. Moderna has ramped up to 1 billion. Three billion more doses could come from the conglomerate organized by AstraZeneca and Oxford, which includes Serum and several other vaccinemakers. Three China-based companies say they can collectively pump out 3 billion doses of their vaccines this year, and Johnson & Johnson hopes to add another 1 billion doses of its single-shot vaccine. Some 850 million doses of the Russian Sputnik V vaccine could come from the Gamaleya Research Institute of Epidemiology and Microbiology and its contract manufacturers. And companies such as Novavax, CureVac, and Clover Biopharmaceuticals all have vaccines in efficacy trials and are hoping for market authorizations in the months ahead.
All told, 14 billion doses could leave factories before the year is over, according to a document written in advance of a March summit about stepping up the production pace. That’s a startling number—before the pandemic, all of the world’s vaccinemakers together produced at most 5.5 billion doses annually—and the document stresses that it’s the best of all scenarios. “We have a set of manufacturing challenges that make it unlikely that we can meet the 14-billion aspiration,” cautions Lurie, who helped organize the meeting.
Raw materials, such as disposable bags that line bioreactors, filters, and cell-culture media, are the biggest challenge. “Raw material inputs have nowhere near kept up with the anticipated demand,” Lurie says. Importing and exporting delays have exacerbated the shortages, and travel bans have made it difficult to move experts around the world to troubleshoot manufacturing snafus. Almost every vaccine producer has failed to deliver on initial promises.
New viral variants that evade vaccine-induced immunity could limit the vaccines’ usefulness, as could safety problems. The AstraZeneca-Oxford shot fell out of favor in South Africa because it failed against a variant, and the European Union plans to phase out the vaccine because of the rare clotting disorder. China’s Sinopharm and Sinovac Biotech vaccines have far lower efficacy in some studies than other products and may require a third shot after 6 months.
Demand for several vaccines could also drop because mRNA vaccines work so well and are easy to modify for new variants. Withholding them from large parts of the world may come to be seen as an injustice, says viral immunologist Lawrence Corey of the Fred Hutchinson Cancer Research Center. “We’re going to be judged in our humanity by how we utilize the technologies,” he says.
MONTHS TO YEARS
Sharing knowledge
Allowing more companies to follow the vaccine recipes developed—and sometimes fiercely protected—by a few could also boost output. AstraZeneca and Oxford provided a license for their vaccine to Serum, and then helped the company learn to manufacture it in India, an intensive process known as technology transfer. Most vaccine companies have shied away from such deals, however.
Several ideas have been floated to change that. In May 2020, WHO launched the COVID-19 Technology Access Pool (C-TAP), a system under which companies with proven products would voluntarily share their know-how and intellectual property (IP). The hope was that new manufacturers could pluck whatever they needed from C-TAP, either at a reasonable fee or for free, to start to make their own products. But C-TAP has yet to attract any participants because, critics say, companies want to hold on to monopolies and see sharing as a threat to profits. That has amplified calls for more drastic steps, including taking away the cornerstone of their IP: the patent.
In October 2020, India and South Africa asked the World Trade Organization (WTO) to issue a broad-reaching waiver for patents and other intellectual properties that pertain to “prevention, containment or treatment of COVID-19.” The idea has made little headway, although proponents were heartened on 5 May when U.S. Trade Representative Katherine Tai issued a statement explicitly supporting a WTO waiver for COVID-19 vaccines. “This is a global health crisis, and the extraordinary circumstances of the COVID-19 pandemic call for extraordinary measures,” Tai said.
The pharmaceutical industry decried the statement, arguing that giving away IP would remove the incentive to innovate. Inexperienced new vaccine manufacturers would also drain the limited supply of raw materials, Pfizer CEO Albert Bourla warned in a recent letter, “putting the safety and security of all at risk.” Moderna CEO Stéphane Bancel said waiving patents is “the wrong question,” because new companies would not be able to produce mRNA vaccines this year or next, “the most critical time of the pandemic.” Although Moderna has promised not to enforce its own COVID-19–related IP rights during the pandemic, making its vaccine still requires licensing deals with other patent holders—including a critical one held by the University of Pennsylvania—and, most important, know-how.
Martin Friede, who helps coordinate vaccine research at WHO, applauds Moderna for waiving its patents but agrees that “the patent by itself is useless.” New companies would still need to set up facilities, train staff, and learn how to produce the vaccines. A patent is a recipe but doesn’t make you a chef, Friede says: “I’ve got a lot of cookbooks in my kitchen, but you wouldn’t want to come to my house if I’m doing the cooking.” He and his colleagues are trying to create a training center where scientists and manufacturers from low- and middle-income countries could learn how to set up the industrial process for mRNA vaccines. “You go back to your facility, and now instead of spending years setting it up, you’re up and running in a matter of months,” he says.
James Love, who runs the nongovernmental organization Knowledge Ecology International, says waiving patents would kick-start the process for many would-be vaccinemakers. “It’s been illegal for a year and a half to even work on these vaccines,” Love says. “Who’s going to invest in developing something where it’s illegal?”
Others say the threat of patent waivers can act as a cudgel, compelling big pharmaceutical companies to share their patents and expertise. IPPPR wrote in its report to WHO that IP waivers should be implemented if companies fail to negotiate voluntary licenses and technology transfers within 3 months.
That has worked in the past, Dybul says: Lifesaving antiretroviral drugs developed in the 1990s long remained too expensive for most HIV-infected people. But the threat that their patents would be steamrolled led Big Pharma to share its knowledge with generic drugmakers, and prices plummeted. To Dybul, the vaccinemakers’ reaction today echoes what drug companies said back then: “Just let us produce, we know how to do it, it’s too complicated, can’t be done.” But he acknowledges that vaccines are more difficult to produce than drugs.
Ideally, Friede says he’d like Pfizer, BioNTech, and Moderna to “play ball with us” and help set up the training facility, but he’s soliciting help from other makers of mRNA vaccines who have their own patent portfolios but don’t yet have regulatory authorization.
YEARS
Building plants worldwide
Freeing up IP could allow existing plants to produce vaccines that are now off-limits. But in the long term, additional manufacturing plants will be needed to serve the needs of the have-nots—not just for this pandemic, but for future ones as well. COVID-19 variants and waning immunity to the virus each could create an annual need for several billion vaccine doses. And if a new disease surfaces, the world could find itself again needing billions of doses of new vaccines.
The inequitable way COVID-19 vaccines have been rolled out underscores the limits of a system that concentrates manufacturing power in a few locations, says Leena Menghaney, who heads Doctors Without Borders’s Access Campaign for South-East Asia. The bulk of COVID-19 vaccine doses so far have been made in the United States, China, India, and Europe—and all have felt pressured to vaccinate their own people first. “Once we’re done with ours we’ll give it to you: That argument is very much in your face and it’s the subtext of the whole conversation,” Menghaney says.
Jeremy Farrar, head of the Wellcome Trust, suggests establishing global enterprise zones that produce vaccines in countries with small populations, such as Senegal, Singapore, Costa Rica, or Rwanda. “They could provide vaccine for their own domestic citizens very quickly and could then be manufacturing hubs for the world,” he says. And he advocates that countries get together and make “at-risk” investments—gambles that may not pay off—in these new plants.
Rwandan President Paul Kagame said at a 13 April African Union conference that his country has already discussed construction of an mRNA plant with several manufacturers. One of them, GreenLight BioSciences, has issued a “blueprint of how to vaccinate the world” with small, modular mRNA plants that it can build in Massachusetts and ship anywhere. A single production room, roughly the size of four shipping containers, could make most of its own raw materials and crank out 17 million doses per month. For about $200 million the company says it could provide a mini–vaccine plant and a “clean room” to house it. The company says one of these could be up and running in as little as 1 year after an agreement is signed. “You could leapfrog our ability to fight pandemics,” Dybul says.
Mark Feinberg, who heads the International Aids Vaccine Initiative, likes the idea, but says it faces many hurdles. Large vaccinemakers have economies of scale, reliable supply chains for raw materials, stringent quality control, and regular inspections, Feinberg says. “Rwanda is an amazing place in terms of public health and commitment to innovation,” he says, but “it’s not like you snap your fingers and all of a sudden there are highly capable people who know every detail about the manufacturing process and can ensure that it’s operating as it should.” It’s also unclear what vaccine plants and staff would do between pandemics.
A year and a half into the pandemic, COVID-19 has shined a bright light on hugely complicated issues. “The challenge that’s being taken on here is unprecedented: You want to vaccinate everyone on the planet. That has never been done before,” Feinberg says. “You might say: If we do this in 3 years that’s an accomplishment that is unprecedented in the history of the human race. But if you actually think about the tragedy that COVID is imposing, that seems like a really long, long time.”