Science's COVID-19 reporting is supported by the Pulitzer Center and the Heising-Simons Foundation.
The backers of Australia's homegrown COVID-19 vaccine candidate today announced a halt to its further development, after some of the first people to receive the vaccine in a safety trial generated antibodies to an unintended target, the AIDS virus. A small fragment of an HIV protein is a component of the vaccine used to add stability to the intended antibody target, the spike protein of the pandemic coronavirus SARS-CoV-2.
Although the added component didn't represent an actual infection with HIV, the vaccine developers and the Australian government concluded a widespread rollout of the candidate would interfere with HIV diagnostic tests and decided not to proceed to larger clinical trials that would have measured its protection against COVID-19. Given the strong efficacy shown by several recent COVID-19 vaccines, it's likely that other candidates still in development will now have a higher bar to clear to move forward.
The "molecular clamp" technology behind the Australian vaccine was developed by researchers at the University of Queensland (UQ) with support from the global nonprofit Coalition for Epidemic Preparedness Innovations (CEPI), which has backed multiple COVID-19 vaccine candidates. CSL Limited, a Melbourne, Australia–based pharmaceutical firm that licensed the UQ candidate, was planning to steer it through phase II and III trials and manufacture it.
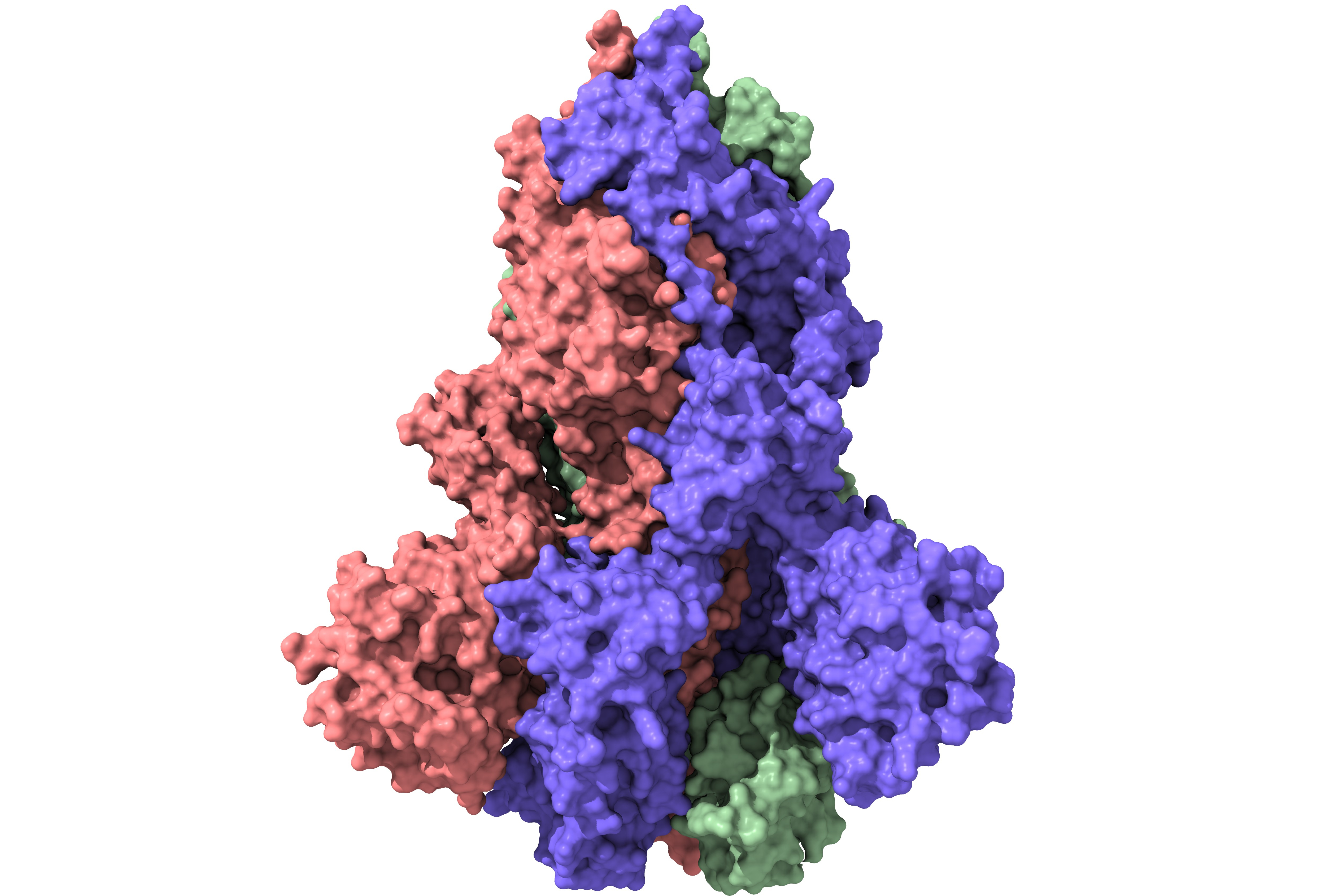
Among current COVID-19 candidate vaccines, "the molecular clamp is a unique approach," says Adam Taylor, a virologist at Griffith University, Gold Coast, who was not affiliated with the vaccine development effort. He explains that like many COVID-19 vaccines, including the several that appear to have clear efficacy, the UQ-CSL candidate presents the SARS-CoV-2 spike so the human body will generate antibodies to the protein.
Spike plays a central role in infecting cells, folding and reconfiguring as it goes through the process of attaching—or fusing—to a receptor protein on a human cell. The molecular clamp approach centers on a prefusion configuration, which exposes parts of spike hidden in its postfusion form and may generate a more robust antibody response. But this conformation of the spike protein is unstable, so the Australian team introduced a bit of molecular material—the molecular clamp—to hold it in position. The UQ team found that a small 80-amino-acid fragment of an HIV protein provided this stability. (Other groups have introduced mutations into the gene for spike to create a more stable form.)
Unfortunately, that HIV fragment also generated antibodies that could confuse diagnostic tests. Researchers recognized the possibility that the HIV component might invoke an immune response. But, "It was unexpected that the levels induced would interfere with certain HIV tests," reads a joint statement announcing the halt of the candidate's development that was posted online by UQ and CSL.
Aside from the problematic HIV protein immune response, the vaccine, given to 216 participants in July, "elicits a robust response towards the virus [SARS-CoV-2] and has a strong safety profile," according to the statement. The phase I safety trial will continue, to see how long antibodies to the HIV protein persist, though indications are that levels are already falling. The university plans to submit the full data for peer-reviewed publication.
Although it might be possible to re-engineer the vaccine, doing so would set back development by a year or so, Paul Young, a UQ virologist who co-leads the vaccine team, noted in the joint statement. He described the phase I results as "really encouraging" and said that "with further work the molecular clamp technology will be a robust platform for future vaccine development." In a statement, CEPI announced it still believes the concepts underlying the vaccine technology "show great promise" and will continue to support work at UQ on the molecular clamp platform.
Australian media reported the government had signed a deal to buy 51 million doses of the UQ-CSL vaccine but that it also has supply agreements for three other potential or proven COVID-19 vaccines. "Vaccine supply in the absence of the UQ vaccine should not be an issue, assuming some of [the others] are successful in their Phase 3 trials," Sanjaya Senanayake, an infectious disease specialist at the Australian National University, said in a statement distributed by the Australian Science Media Center.
COVID-19 Update: The connection between local and global issues–the Pulitzer Center's long standing mantra–has, sadly, never been more evident. We are uniquely positioned to serve the journalists, news media organizations, schools, and universities we partner with by continuing to advance our core mission: enabling great journalism and education about underreported and systemic issues that resonate now–and continue to have relevance in times ahead. We believe that this is a moment for decisive action. Learn more about the steps we are taking.