Science’s COVID-19 reporting is supported by the Pulitzer Center and the Heising-Simons Foundation.
Today’s dramatic news that Moderna’s COVID-19 vaccine might work as well as one made by Pfizer and BioNTech means the world could have two powerful weapons to fight the COVID-19 pandemic. Now, the next hot vaccine topic is, well, heat. Both vaccines use a novel technology—strands of messenger RNA (mRNA), held within lipid particles—that is vulnerable to degradation at room temperature and requires doses to be frozen for transportation, then thawed for use.
That’s where the Moderna vaccine may have an edge: Unlike Pfizer’s and BioNTech’s offering, it does not have to be stored at –70°C, but can tolerate a much warmer –20°C, which is standard for most hospital and pharmacy freezers. That difference means Moderna’s vaccine should be easier to distribute and store, particularly in the rural United States and developing countries that lack ultracold freezers. Moderna says years of development work enabled its vaccine to be stored at higher temperatures, but last week another mRNA vaccine company announced it is testing a COVID-19 vaccine that early studies suggest can survive at the even warmer temperatures of 2°C to 8°C found in refrigerators.
Many types of vaccines must be stored and transported frozen, via a cold supply chain. Public health officials have even found ways to keep a vaccine ultracold, between –60°C to –80°C, in places like sub-Saharan Africa. There, for the past 5 years, a high-tech thermos called Arktek has helped distribute Ebola vaccines that must be kept ultracold. “In all likelihood, we’ll need a wide range of supply chain tools” to distribute COVID-19 vaccines, says Daniel Lieberman, a mechanical engineer with Global Health Labs in Seattle, a nonprofit created by the Bill & Melinda Gates Foundation and by the private office of Bill Gates (who also funded Arktek’s development). Still, relying on an ultracold chain is expensive, and in some places it may make more sense to distribute a vaccine that can tolerate warmer temperatures even if it’s less effective.
Both the Moderna and Pfizer/BioNTech vaccines give the body’s cells an mRNA template for making the spike protein of SARS-CoV-2, the virus that causes COVID-19. The protein then moves to the cell’s surface and triggers an immune response. This mRNA is relatively fragile compared with the proteins or protein fragments that often make up conventional vaccines, and it cleaves easily at room temperature, says Alana Gerhardt, who studies vaccine product development at the nonprofit Infectious Disease Research Institute (IDRI) in Seattle. Also, enzymes called ribonucleases that chew up mRNAs “are everywhere, even in the controlled environment of the lab,” from sources such as lab workers’ breath and skin, Gerhardt says.
The companies give the mRNA some protection during production and storage by inserting it into a carrier, a fatlike substance called a lipid nanoparticle. The lipid also shields the mRNA from enzymes in the blood once it has been injected. But the nanoparticle is deliberately designed to slowly degrade, so it won’t build up in the liver and cause harm, says Massachusetts Institute of Technology geneticist and chemical engineer Daniel Anderson.
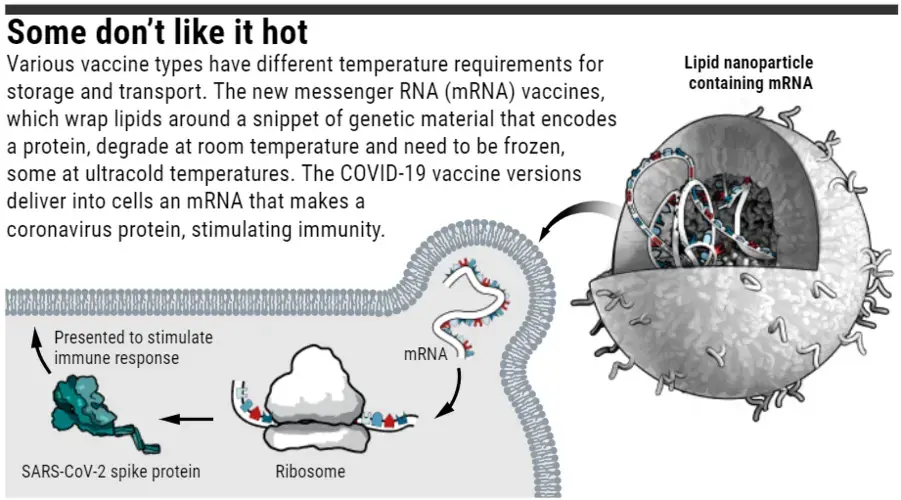
Although the companies’ exact formulations are proprietary, Pfizer and BioNTech have so far said their vaccine has to be kept ultracold. But Barney Graham, a vaccine researcher at the U.S. National Institute of Allergy and Infectious Diseases who designed the mRNA used by both Moderna and Pfizer/BioNTech, says the temperature requirements are actually unclear. Pfizer and BioNtech started with –70°C when they first asked regulators to test their vaccine in humans, and Graham says the companies might ultimately be able to document that the mRNA survives warmer temperatures.
In the meantime, at sites in Kalamazoo, Michigan, and Puurs, Belgium, Pfizer will pack shipments of 200 to 1000 vials each containing five doses in insulated boxes with thermal sensors on dry ice to provide the necessary chill. Pharmacies and doctor’s offices that lack $15,000 ultracold freezers can store it in the thermal box for about 2 weeks by refilling the dry ice every 5 days; once removed, the vaccine can be refrigerated for 5 days. Moderna’s vaccine, however, is stable for 6 months at –20°C, or in a standard freezer, and the company announced today its product can be kept at normal refrigerator temperatures for up to 30 days, longer than the 7 days initially expected.
The German company CureVac is lagging behind in the mRNA COVID-19 vaccine race, but announced last week that its candidate is stable for 3 months at 5°C. Unlike the Moderna and Pfizer/BioNTech vaccines, CureVac’s mRNA does not have a modification to one of its four building blocks, a nucleoside called uridine, which the company says allows it to pack more tightly inside the nanoparticle. “Our hypothesis is that the more compact the mRNA is, the less susceptible it is to degradation,” CureVac spokesperson Sarah Fakih says. The company expects to launch a 30,000-person trial to test the vaccine’s efficacy by the end of this year.
Even a vaccine shipped and stored at –20°C, like Moderna’s, poses challenges in developing countries, where electricity for freezers can be unreliable and dry ice scarce. But frozen vaccines don’t have to be a deal breaker, as shown by the Ebola vaccine, which has been shipped in Arktek containers to different sites in the Democratic Republic of the Congo to vaccinate about 400,000 people. Ultracold freezers in large cities were still needed to store the vaccine before it was distributed and to chill the cold packs used to keep Arkteks cooled, notes Prashant Yadav of the Center for Global Development. That meant there remained a requirement for reliable electricity with backup sources. “The key thing is to have a cold chain primary storage point,” Yadav says.
The Arkteks are expensive—about $2000 each—but they’re reusable and can keep vaccines and dry ice or alcohol or salt-based cold packs chilled for much longer than an insulated box, Lieberman notes. “I see the Arktek being valuable anywhere where you want the vaccines to stay cold for days or weeks without a freezer,” Lieberman says. The Gates Foundation’s global delivery program is readying to ramp up production of the Arktek by the Chinese company that makes it, if needed for COVID-19 vaccines, Global Health Labs says.
Another option is to freeze-dry the mRNA vaccines; they would then be reconstituted with water at the delivery point. (Other vaccines already do this.) Pfizer says it is working on such a powderized form of its vaccine. Regulators would likely require evidence to show the reconstituted vaccine works as well as the liquid vaccine, says IDRI’s Corey Casper.
Several other COVID-19 vaccines now in efficacy trials won’t require freezer storage either. If they work as well as the mRNA vaccines, there will be no need for a –20°C or –70°C cold chain in resource-restrained settings, Yadav says. But if these vaccines aren’t as effective—say only 70%, compared with 90% for an mRNA vaccine—Yadav says that might tip the scale toward deploying mRNA vaccines, despite the costs and challenges.
With reporting by Jon Cohen.
COVID-19 Update: The connection between local and global issues–the Pulitzer Center's long standing mantra–has, sadly, never been more evident. We are uniquely positioned to serve the journalists, news media organizations, schools, and universities we partner with by continuing to advance our core mission: enabling great journalism and education about underreported and systemic issues that resonate now–and continue to have relevance in times ahead. We believe that this is a moment for decisive action. Learn more about the steps we are taking.